Oxide Formation
Oxide formation in concentrated refractory alloy
This project is led by Vinod Sarky, who is a Master’s student in our research group.
In our earlier work
We found that the first nearest neighbors (1NN) has the largest influence on the stability of oxygen interstitial in concenterated alloys. We studied 24 equiatomic binary alloys made from Ti, Zr, Hf (Group IV), V, Nb, Ta (Group V), Mo, W (Group VI), and Re (Group VII). Through our first-principles DFT calculations, we found that if the oxygen (at the octahedral interstitial position) is surrounded by an element having high susceptiblity for oxidation then the system behave as if the oxygen is in the bulk of that material. It means that the influence of third and fourth nearest neighbors is minimal on the oxygen interstitial stability. This work is published in the Journal of Alloys and Compounds
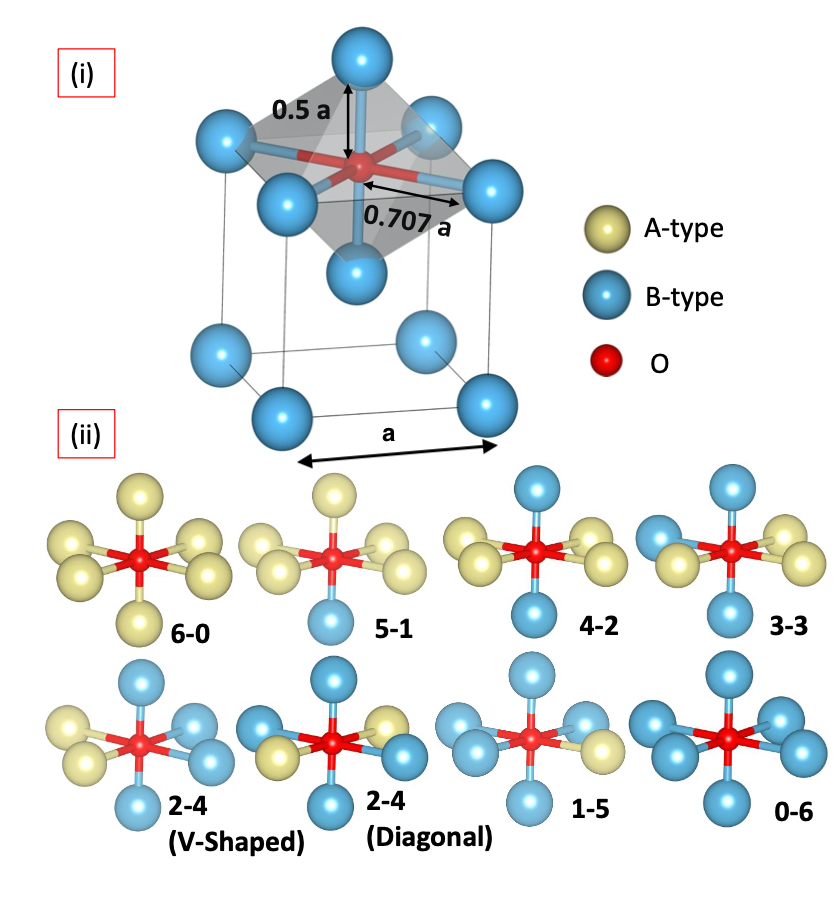
The first step in oxidation of an alloy is the generation of a stable oxygen interstitial at an energetically favorable site. If we are able to “engineer” better local chemistry variation in the alloy crystal then the oxygen interstitial can be made unstable in the alloy, i.e., we will be able to design oxidation resistant refractory alloys for high-temperature applications.
Vinod has developed a code to calcualte the list of nearest neighbors (NN) based on cut-off radius.